Phosphinoborylenes as stable sources of fleeting borylenes†
Abstract
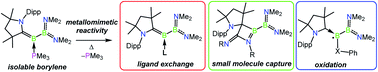
Base-stabilised borylenes that mimic the ability of transition metals to bind and activate inert substrates have attracted significant attention in recent years. However, such species are typically highly reactive and fleeting, and often cannot be isolated at ambient temperature. Herein, we describe a readily accessible trimethylphosphine-stabilised borylborylene which was found to possess a labile P–B bond that reversibly cleaves upon gentle heating. Exchange of the labile phosphine with other nucleophiles (CO, isocyanide, 4-dimethylaminopyridine) was investigated, and the binding strength of a range of potential borylene “ligands” has been evaluated computationally. The room-temperature-stable PMe3-bound borylenes were subsequently applied to novel bond activations including [2 + 2] cycloaddition with carbodiimides and the reduction of dichalcogenides, revealing that PMe3-stabilised borylenes can effectively behave as stable sources of the analogous fleeting dicoordinate species under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...