Diverse strategies for transition metal catalyzed distal C(sp3)–H functionalizations
Abstract
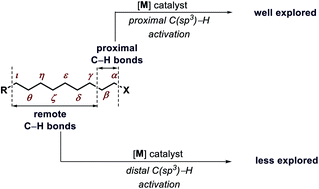
Transition metal catalyzed C(sp3)–H functionalization is a rapidly growing field. Despite severe challenges, distal C–H functionalizations of aliphatic molecules by overriding proximal positions have witnessed tremendous progress. While usage of stoichiometric directing groups played a crucial role, reactions with catalytic transient directing groups or methods without any directing groups are gaining more attention due to their practicality. Various innovative strategies, slowly but steadily, circumvented issues related to remote functionalizations of aliphatic molecules. A systematic compilation has been presented here to provide insights into the recent developments and future challenges in the field. The Present perspective is expected to open up a new dimension and provide an avenue for deep insights into the distal C(sp3)–H functionalizations that could be applied routinely in various pharmaceutical and agrochemical industries.
- This article is part of the themed collection: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020


 Please wait while we load your content...
Please wait while we load your content...