Bioorthogonal release of anticancer drugs via gold-triggered 2-alkynylbenzamide cyclization†
Abstract
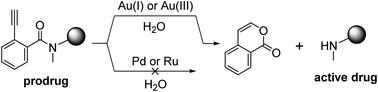
Metal-based uncaging of biomolecules has become an emerging approach for in vivo applications, which is largely due to the advantageous bioorthogonality of abiotic transition metals. Adding to the library of metal-cleavable protecting groups, this work introduces the 2-alkynylbenzamide (Ayba) moiety for the gold-triggered release of secondary amines under mild and physiological conditions. Studies were further performed to highlight some intrinsic benefits of the Ayba protecting group, which are (1) its amenable nature to derivatization for manipulating prodrug properties, and (2) its orthogonality with other commonly used transition metals like palladium and ruthenium. With a focus on highlighting its application for anticancer drug therapies, this study successfully shows that gold-triggered conversion of Ayba-protected prodrugs into bioactive anticancer drugs (i.e. doxorubicin, endoxifen) can proceed effectively in cell-based assays.


 Please wait while we load your content...
Please wait while we load your content...