Uncovering biosynthetic relationships between antifungal nonadrides and octadrides†
Abstract
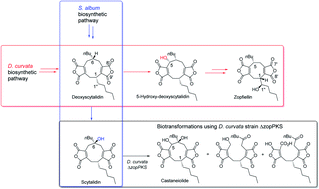
Maleidrides are a class of bioactive secondary metabolites unique to filamentous fungi, which contain one or more maleic anhydrides fused to a 7-, 8- or 9- membered carbocycle (named heptadrides, octadrides and nonadrides respectively). Herein structural and biosynthetic studies on the antifungal octadride, zopfiellin, and nonadrides scytalidin, deoxyscytalidin and castaneiolide are described. A combination of genome sequencing, bioinformatic analyses, gene disruptions, biotransformations, isotopic feeding studies, NMR and X-ray crystallography revealed that they share a common biosynthetic pathway, diverging only after the nonadride deoxyscytalidin. 5-Hydroxylation of deoxyscytalidin occurs prior to ring contraction in the zopfiellin pathway of Diffractella curvata. In Scytalidium album, 6-hydroxylation – confirmed as being catalysed by the α-ketoglutarate dependent oxidoreductase ScyL2 – converts deoxyscytalidin to scytalidin, in the final step in the scytalidin pathway. Feeding scytalidin to a zopfiellin PKS knockout strain led to the production of the nonadride castaneiolide and two novel ring-open maleidrides.


 Please wait while we load your content...
Please wait while we load your content...