3-Aminooxetanes: versatile 1,3-amphoteric molecules for intermolecular annulation reactions†
Abstract
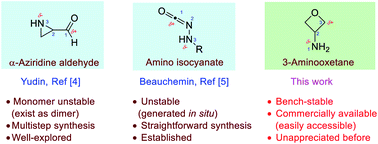
Despite the versatility of amphoteric molecules, stable and easily accessible ones are still limitedly known. As a result, the discovery of new amphoteric reactivity remains highly desirable. Herein we introduce 3-aminooxetanes as a new family of stable and readily available 1,3-amphoteric molecules and systematically demonstrated their amphoteric reactivity toward polarized π-systems in a diverse range of intermolecular [3 + 2] annulations. These reactions not only enrich the reactivity of oxetanes, but also provide convergent access to valuable heterocycles.


 Please wait while we load your content...
Please wait while we load your content...