A molecular approach to rationally constructing specific fluorogenic substrates for the detection of acetylcholinesterase activity in live cells, mice brains and tissues†
Abstract
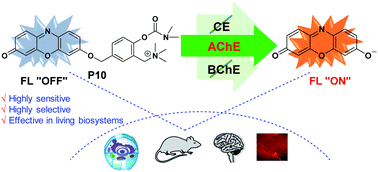
Acetylcholinesterase (AChE) is an extremely critical hydrolase tightly associated with neurological diseases. Currently, developing specific substrates for imaging AChE activity still remains a great challenge due to the interference from butyrylcholinesterase (BChE) and carboxylesterase (CE). Herein, we propose an approach to designing specific substrates for AChE detection by combining dimethylcarbamate choline with a self-immolative scaffold. The representative P10 can effectively eliminate the interference from CE and BChE. The high specificity of P10 has been proved via imaging AChE activity in cells. Moreover, P10 can also be used to successfully map AChE activity in different regions of a normal mouse brain, which may provide important data for AChE evaluation in clinical studies. Such a rational and effective approach can also provide a solid basis for designing probes with different properties to study AChE in biosystems and another way to design specific substrates for other enzymes.


 Please wait while we load your content...
Please wait while we load your content...