Photocatalytic redox-neutral hydroxyalkylation of N-heteroaromatics with aldehydes†
Abstract
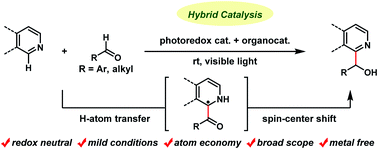
Hydroxyalkylation of N-heteroaromatics with aldehydes was achieved using a binary hybrid catalyst system comprising an acridinium photoredox catalyst and a thiophosphoric acid organocatalyst. The reaction proceeded through the following sequence: (1) photoredox-catalyzed single-electron oxidation of a thiophosphoric acid catalyst to generate a thiyl radical, (2) cleavage of the formyl C–H bond of the aldehyde substrates by a thiyl radical acting as a hydrogen atom transfer catalyst to generate acyl radicals, (3) Minisci-type addition of the resulting acyl radicals to N-heteroaromatics, and (4) a spin-center shift, photoredox-catalyzed single-electron reduction, and protonation to produce secondary alcohol products. This metal-free hybrid catalysis proceeded under mild conditions for a wide range of substrates, including isoquinolines, quinolines, and pyridines as N-heteroaromatics, as well as both aromatic and aliphatic aldehydes, and tolerated various functional groups. The reaction was applicable to late-stage derivatization of drugs and their leads.


 Please wait while we load your content...
Please wait while we load your content...