Key aurophilic motif for robust quantum-tunneling-based characterization of a nucleoside analogue marker†
Abstract
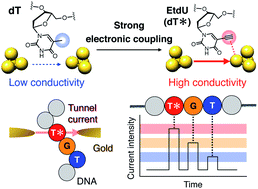
A quantum sequencer offers a scalable electrical platform for single-molecule analysis of genomic events. A thymidine (dT) analog exhibiting uniquely high single-molecule conductance is a key element in capturing DNA synthesis dynamics by serving as a decodable marker for enzymatic labeling of nascent strands. However, the current design strategies of dT analogs that focus on their molecular orbital energy levels require bulky chemical modifications to extend the π-conjugation, which hinders polymerase recognition. We report herein a polymerase-compatible dT analog that is highly identifiable in quantum sequencing. An ethynyl group is introduced as a small gold-binding motif to differentiate the nucleobase–gold electronic coupling, which has been an overlooked factor in modifying nucleobase conductance. The resulting C5-ethynyl-2′-deoxyuridine exhibits characteristic signal profiles that allowed its correct identification at a 93% rate while maintaining polymerase compatibility. This study would expand the applicability of quantum sequencing by demonstrating a robust nucleoside marker with high identifiability.


 Please wait while we load your content...
Please wait while we load your content...