Merging C(sp3)–H activation with DNA-encoding†
Abstract
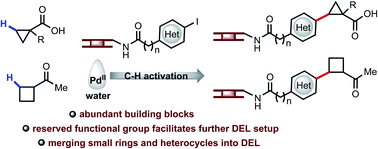
DNA-encoded library (DEL) technology has the potential to dramatically expedite hit identification in drug discovery owing to its ability to perform protein affinity selection with millions or billions of molecules in a few experiments. To expand the molecular diversity of DEL, it is critical to develop different types of DNA-encoded transformations that produce billions of molecules with distinct molecular scaffolds. Sequential functionalization of multiple C–H bonds provides a unique avenue for creating diversity and complexity from simple starting materials. However, the use of water as solvent, the presence of DNA, and the extremely low concentration of DNA-encoded coupling partners (0.001 M) have hampered the development of DNA-encoded C(sp3)–H activation reactions. Herein, we report the realization of palladium-catalyzed C(sp3)–H arylation of aliphatic carboxylic acids, amides and ketones with DNA-encoded aryl iodides in water. Notably, the present method enables the use of alternative sets of monofunctional building blocks, providing a linchpin to facilitate further setup for DELs. Furthermore, the C–H arylation chemistry enabled the on-DNA synthesis of structurally-diverse scaffolds containing enriched C(sp3) character, chiral centers, cyclopropane, cyclobutane, and heterocycles.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...