Self-assembly of cyclic hexamers of γ-cyclodextrin in a metallosupramolecular framework with d-penicillamine†
Abstract
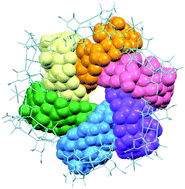
Cyclodextrins are widely used cyclic oligosaccharides of D-glucose whose hydrophilic exterior is covered by hydroxyl groups and whose hydrophobic interior is surrounded by lipophilic moieties. Because of this structural feature, cyclodextrin molecules commonly aggregate into dimensional structures via intermolecular hydrogen bonds, and their aggregation into closed oligomeric architectures has been achieved only via the attachment of functional substituent groups to the cyclodextrin rings. Here, we report the first structurally characterized self-assembly of non-substituted γ-cyclodextrin molecules into cyclic hexamers, which was realized in a chiral coordination framework composed of 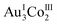 complex-anions with D-penicillamine rather than L- or DL-penicillamine. The self-assembly is accompanied by the 3D-to-2D structural transformation of porous coordination frameworks to form helical hexagonal cavities that accommodate helical γ-cyclodextrin hexamers. This finding provides new insight into the development of cyclodextrin chemistry and host–guest chemistry based on chiral recognition and crystal engineering processes.
complex-anions with D-penicillamine rather than L- or DL-penicillamine. The self-assembly is accompanied by the 3D-to-2D structural transformation of porous coordination frameworks to form helical hexagonal cavities that accommodate helical γ-cyclodextrin hexamers. This finding provides new insight into the development of cyclodextrin chemistry and host–guest chemistry based on chiral recognition and crystal engineering processes.


 Please wait while we load your content...
Please wait while we load your content...