Aluminum-catalyzed tunable halodefluorination of trifluoromethyl- and difluoroalkyl-substituted olefins†
Abstract
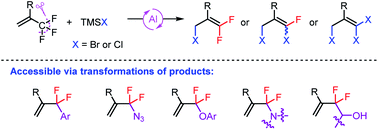
Herein, we report unprecedented aluminum-catalyzed halodefluorination reactions of trifluoromethyl- and difluoroalkyl-substituted olefins with bromo- or chlorotrimethylsilane. The interesting feature of these reactions is that one, two, or three fluorine atoms can be selectively replaced with bromine or chlorine atoms by modification of the reaction conditions. The generated products can undergo a variety of subsequent transformations, thus constituting a valuable stock of building blocks for installing fluorine-containing olefin motifs in other molecules.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...