Regiocontrolled dimerization of asymmetric diazaheptacene derivatives toward X-shaped porous semiconductors†
Abstract
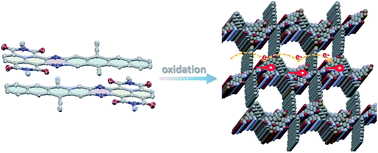
Conformationally rigid X-shaped PAHs are attracting interest due to their self-assembly into unique networks and as models to study through-space exciton and charge delocalization in one single molecule. We report here the synthesis of X-shaped PAHs by dimerization of diazaheptacene diimides. The diimide groups are employed to effectively direct the self-assembly into antiparallel dimer aggregates, which assist the compounds to undergo a regiocontrolled [4 + 4] dimerization, leading to an X-shaped conformation bearing electron-poor and -rich subunits. The resulting PAHs are found to pack in 2D layers with large open channels and infinite π⋯π arrays. Furthermore, these highly crystalline porous materials serve as electron-transporting materials in OFETs due to the long-range π-stacked arrays in the layers. This work presents a potentially generalizable strategy, which may provide a unique class of porous semiconductors for organic devices, taking advantage of their open channels.


 Please wait while we load your content...
Please wait while we load your content...