Fungal siderophore biosynthesis catalysed by an iterative nonribosomal peptide synthetase†
Abstract
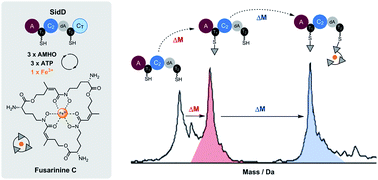
Siderophores play a vital role in the viability of fungi and are essential for the virulence of many pathogenic fungal species. Despite their importance in fungal physiology and pathogenesis, the programming rule of siderophore assembly by fungal nonribosomal peptide synthetases (NRPSs) remains unresolved. Here, we report the characterization of the bimodular fungal NRPS, SidD, responsible for construction of the extracellular siderophore fusarinine C. The use of intact protein mass spectrometry, together with in vitro biochemical assays of native and dissected enzymes, provided snapshots of individual biosynthetic steps during NPRS catalysis. The adenylation and condensation domain of SidD can iteratively load and condense the amino acid building block cis-AMHO, respectively, to synthesize fusarinine C. Our study showcases the iterative programming features of fungal siderophore-producing NRPSs.


 Please wait while we load your content...
Please wait while we load your content...