Acyclic diaminocarbene-based Thiele, Chichibabin, and Müller hydrocarbons†
Abstract
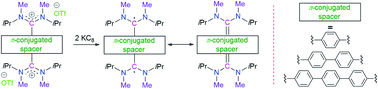
Thiele, Chichibabin and Müller hydrocarbons are considered as classical Kekulé diradicaloids. Herein we report the synthesis and characterization of acyclic diaminocarbene (ADC)-based Thiele, Chichibabin, and Müller hydrocarbons. The calculated singlet–triplet energy gaps are ΔES–T = −27.96, −3.70, −0.37 kcal mol−1, respectively, and gradually decrease with the increasing length of the π-conjugated spacer (p-phenylene vs. p,p′-biphenylene vs. p,p′′-terphenylene) between the two ADC-scaffolds. In agreement with the calculations, we also experimentally observed the enhancement of paramagnetic diradical character as a function of the length of the π-conjugated spacer. ADC-based Thiele's hydrocarbon is EPR silent and exhibits very well resolved NMR spectra, whereas ADC-based Müller's hydrocarbon displays EPR signals and featureless NMR spectra at room temperature. The spacer also has a strong influence on the UV-Vis-NIR spectra of these compounds. Considering that our methodology is modular, these results provide a convenient platform for the synthesis of an electronically modified new class of carbon-centered Kekulé diradicaloids.


 Please wait while we load your content...
Please wait while we load your content...