Synthesis of unsymmetrical sulfamides and polysulfamides via SuFEx click chemistry†
Abstract
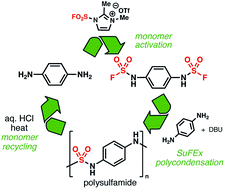
As hydrogen-bond donors and acceptors, N,N′-disubstituted sulfamides have been used in a range of applications from medicinal chemistry to anion-binding catalysis. However, compared to ureas or thioureas, the utilization of this unique moiety remains marginal, in part because of a lack of general synthetic methods to access unsymmetrical sulfamides. Specifically, polysulfamides are a virtually unknown type of polymer despite their potential utility in non-covalent dynamic networks, an intense area of research in materials science. We report herein a practical and efficient process to prepare unsymmetrical sulfamides via Sulfur(VI)-Fluoride Exchange (SuFEx) click chemistry. This process was then applied to synthesize polysulfamides. Thermal analysis showed that this family of polymers possess high thermal stability and tunable glass transition temperatures. Finally, hydrolysis studies indicated that aromatic polysulfamides could be recycled back to their constituting monomers at the end of their life cycle.
- This article is part of the themed collections: Bioorthogonal and click chemistry: Celebrating the 2022 Nobel Prize in Chemistry and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...