Triple the fun: tris(ferrocenyl)arene-based gold(i) complexes for redox-switchable catalysis†
Abstract
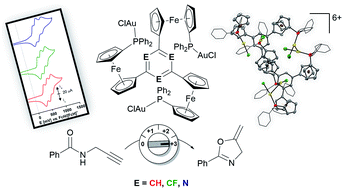
The modular syntheses of C3-symmetric tris(ferrocenyl)arene-based tris-phosphanes and their homotrinuclear gold(I) complexes are reported. Choosing the arene core allows fine-tuning of the exact oxidation potentials and thus tailoring of the electrochemical response. The tris[chloridogold(I)] complexes were investigated in the catalytic ring-closing isomerisation of N-(2-propyn-1-yl)benzamide, showing cooperative behaviour vs. a mononuclear chloridogold(I) complex. Adding one, two, or three equivalents of 1,1′-diacetylferrocenium[tetrakis(perfluoro-tert-butoxy)aluminate] as an oxidant during the catalytic reaction (in situ) resulted in a distinct, stepwise influence on the resulting catalytic rates. Isolation of the oxidised species is possible, and using them as (pre-)catalysts (ex situ oxidation) confirmed the activity trend. Proving the intactness of the P–Au–Cl motif during oxidation, the tri-oxidised benzene-based complex has been structurally characterised.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...