Quantifying internal charge transfer and mixed ion-electron transfer in conjugated radical polymers†
Abstract
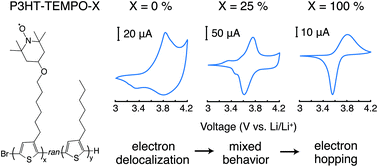
Macromolecular radicals are receiving growing interest as functional materials in energy storage devices and in electronics. With the need for enhanced conductivity, researchers have turned to macromolecular radicals bearing conjugated backbones, but results thus far have yielded conjugated radical polymers that are inferior in comparison to their non-conjugated partners. The emerging explanation is that the radical unit and the conjugated backbone (both being redox active) transfer electrons between each other, essentially “quenching” conductivity or capacity. Here, the internal charge transfer process is quantified using a polythiophene loaded with 0, 25, or 100% nitroxide radicals (2,2,6,6-tetramethyl-1-piperidinyloxy [TEMPO]). Importantly, deconvolution of the cyclic voltammograms shows mixed faradaic and non-faradaic contributions that contribute to the internal charge transfer process. Further, mixed ion-electron transfer is determined for the 100% TEMPO-loaded conjugated radical polymer, from which it is estimated that one triflate anion and one propylene carbone molecule are exchanged for every electron. Although these findings indicate the reason behind their poor conductivity and capacity, they point to how these materials might be used as voltage regulators in the future.


 Please wait while we load your content...
Please wait while we load your content...