Controlling cyclization pathways in palladium(ii)-catalyzed intramolecular alkene hydro-functionalization via substrate directivity†
Abstract
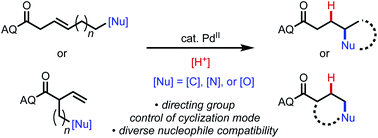
We report a series of palladium(II)-catalyzed, intramolecular alkene hydrofunctionalization reactions with carbon, nitrogen, and oxygen nucleophiles to form five- and six-membered carbo- and heterocycles. In these reactions, the presence of a proximal bidentate directing group controls the cyclization pathway, dictating the ring size that is generated, even in cases that are disfavored based on Baldwin's rules and in cases where there is an inherent preference for an alternative pathway. DFT studies shed light on the origins of pathway selectivity in these processes.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...