Development of photo- and chemo-stable near-infrared-emitting dyes: linear-shape benzo-rosol and its derivatives as unique ratiometric bioimaging platforms†
Abstract
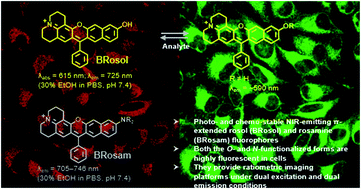
Microscopic imaging aided with fluorescent probes has revolutionized our understanding of biological systems. Organic fluorophores and probes thus continue to evolve for bioimaging applications. Fluorophores such as cyanines and hemicyanines emit in the near-infrared (NIR) region and thus allow deeper imaging with minimal autofluorescence; however, they show limited photo- and chemo-stability, demanding new robust NIR fluorophores. Such photo- and chemo-stable NIR fluorophores, linear-shape π-extended rosol and rosamine analogues, are disclosed here which provide bright fluorescence images in cells as well as in tissues by confocal laser-scanning microscopy. Furthermore, they offer unique ratiometric imaging platforms for activatable probes with dual excitation and dual emission capability, as demonstrated with a 2,4-dinitrophenyl ether derivative of benzo-rosol.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...