Surface site density and utilization of platinum group metal (PGM)-free Fe–NC and FeNi–NC electrocatalysts for the oxygen reduction reaction†
Abstract
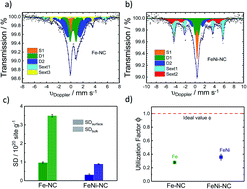
Pyrolyzed iron-based platinum group metal (PGM)-free nitrogen-doped single site carbon catalysts (Fe–NC) are possible alternatives to platinum-based carbon catalysts for the oxygen reduction reaction (ORR). Bimetallic PGM-free M1M2–NC catalysts and their active sites, however, have been poorly studied to date. The present study explores the active accessible sites of mono- and bimetallic Fe–NC and FeNi–NC catalysts. Combining CO cryo chemisorption, X-ray absorption and 57Fe Mössbauer spectroscopy, we evaluate the number and chemical state of metal sites at the surface of the catalysts along with an estimate of their dispersion and utilization. Fe L3,2-edge X-ray adsorption spectra, Mössbauer spectra and CO desorption all suggested an essentially identical nature of Fe sites in both monometallic Fe–NC and bimetallic FeNi–NC; however, Ni blocks the formation of active sites during the pyrolysis and thus causes a sharp reduction in the accessible metal site density, while with only a minor direct participation as a catalytic site in the final catalyst. We also use the site density utilization factor, ϕSDsurface/bulk, as a measure of the metal site dispersion in PGM-free ORR catalysts. ϕSDsurface/bulk enables a quantitative evaluation and comparison of distinct catalyst synthesis routes in terms of their ratio of accessible metal sites. It gives guidance for further optimization of the accessible site density of M–NC catalysts.
- This article is part of the themed collection: Editor’s Choice – Jinlong Gong


 Please wait while we load your content...
Please wait while we load your content...