Fast protein analysis enabled by high-temperature hydrolysis†
Abstract
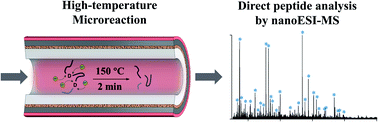
While the bottom-up protein analysis serves as a mainstream method for biological studies, its efficiency is limited by the time-consuming process for enzymatic digestion or hydrolysis as well as the post-digestion treatment prior to mass spectrometry analysis. In this work, we developed an enzyme-free microreaction system for fast and selective hydrolysis of proteins, and a direct analysis of the protein digests was achieved by nanoESI (electrospray ionization) mass spectrometry. Using the microreactor, proteins in aqueous solution could be selectively hydrolyzed at the aspartyl sites within 2 min at high temperatures (∼150 °C). Being free of salts, the protein digest solution could be directly analyzed using a mass spectrometer with nanoESI without further purification or post-digestion treatment. This method has been validated for the analysis of a variety of proteins with molecular weights ranging from 8.5 to 67 kDa. With introduction of a reducing agent into the protein solutions, fast cleavage of disulfide bonds was also achieved along with high-temperature hydrolysis, allowing for fast analysis of large proteins such as bovine serum albumin. The high-temperature microreaction system was also used with a miniature mass spectrometer for the determination of highly specific peptides from Mycobacterium tuberculosis antigens, showing its potential for point-of-care analysis of protein biomarkers.


 Please wait while we load your content...
Please wait while we load your content...