Extension of the Simmons–Smith reaction to metal-carbynes: efficient synthesis of metallacyclopropenes with σ-aromaticity†
Abstract
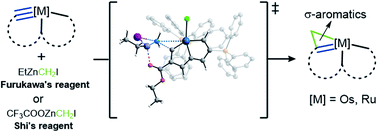
The Simmons–Smith reaction offers a direct route for conversion of an alkene into a cyclopropane with a zinc carbenoid as the active intermediate. Zinc carbenoids, however, have never delivered a methylene unit to substrates with metal–carbon multiple bonds. Herein, we describe this type of reaction and the construction of three-membered rings has now been applied in organometallic systems by combining classical zinc carbenoid reagents with a range of structurally and electronically diverse metal carbynes. A variety of metallacyclopropene derivatives prepared in this way represent rare examples with σ-aromaticity in an unsaturated three-membered ring. The structures of such products are supported by experimental observations and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...