Selective α,δ-hydrocarboxylation of conjugated dienes utilizing CO2 and electrosynthesis†
Abstract
To date the majority of diene carboxylation processes afford the α,δ-dicarboxylated product, the selective mono-carboxylation of dienes is a significant challenge and the major product reported under transition metal catalysis arises from carboxylation at the α-carbon. Herein we report a new electrosynthetic approach, that does not rely on a sacrificial electrode, the reported method allows unprecedented direct access to carboxylic acids derived from dienes at the δ-position. In addition, the α,δ-dicarboxylic acid or the α,δ-reduced alkene can be easily accessed by simple modification of the reaction conditions.
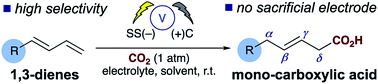
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...