N-Heterocyclic carbene based catalytic platform for Hauser–Kraus annulations†
Abstract
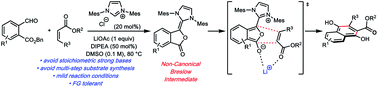
The venerable Hauser–Kraus annulation is an effective and convergent method for generating oxygenated polycyclic aromatic compounds. Despite its application in complex molecule synthesis, the harsh and strongly basic conditions can limit its utility in more functionalized molecular settings. We have developed the first catalytic Hauser–Kraus annulation based on N-heterocyclic carbene catalysis that proceeds under milder conditions. We demonstrate the scope of the transformation in the presence of several functional groups. We also propose a concerted mechanism for the annulation that proceeds through a non-canonical Breslow intermediate.
- This article is part of the themed collection: New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...