Total synthesis of dimeric Securinega alkaloids (−)-flueggenines D and I†
Abstract
We describe the total synthesis of (−)-flueggenines D and I. This features the first total synthesis of dimeric Securinega alkaloids with a C(α)–C(δ′) connectivity between two monomeric units. The key dimerization was enabled by a sequence that involves Stille reaction and conjugate reduction. The high chemofidelity of the Stille reaction enabled us to assemble two structurally complex fragments that could not be connected by other methods. Stereochemical flexibility and controllability at the δ′-junction of the dimeric intermediate render our synthetic strategy broadly applicable to the synthesis of other high-order Securinega alkaloids.
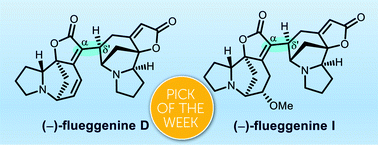
- This article is part of the themed collections: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS) and 2020 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...