Site-selective aromatic C–H λ3-iodanation with a cyclic iodine(iii) electrophile in solution and solid phases†
Abstract
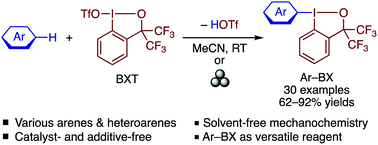
An efficient and site-selective aromatic C–H λ3-iodanation reaction is achieved using benziodoxole triflate (BXT) as an electrophile under room temperature conditions. The reaction tolerates a variety of electron-rich arenes and heteroarenes to afford the corresponding arylbenziodoxoles in moderate to good yields. The reaction can also be performed mechanochemically by grinding a mixture of solid arenes and BXT under solvent-free conditions. The arylbenziodoxoles can be used for various C–C and C–heteroatom bond formations, and are also amenable to further modification by electrophilic halogenation. DFT calculations suggested that the present reaction proceeds via a concerted λ3-iodanation–deprotonation transition state, where the triflate anion acts as an internal base.


 Please wait while we load your content...
Please wait while we load your content...