Electrostatics does not dictate the slip-stacked arrangement of aromatic π–π interactions†
Abstract
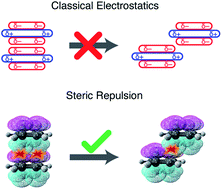
Benzene dimer has long been an archetype for π-stacking. According to the Hunter–Sanders model, quadrupolar electrostatics favors an edge-to-face CH⋯π geometry but competes with London dispersion that favors cofacial π-stacking, with a compromise “slip-stacked” structure emerging as the minimum-energy geometry. This model is based on classical electrostatics, however, and neglects charge penetration. A fully quantum-mechanical analysis, presented here, demonstrates that electrostatics actually exerts very little influence on the conformational landscape of (C6H6)2. Electrostatics also cannot explain the slip-stacked arrangement of C6H6⋯C6F6, where the sign of the quadrupolar interaction is reversed. Instead, the slip-stacked geometry emerges in both systems due to competition between dispersion and Pauli repulsion, with electrostatics as an ambivalent spectator. This revised interpretation helps to rationalize the persistence of offset π-stacking in larger polycyclic aromatic hydrocarbons and across the highly varied electrostatic environments that characterize π–π interactions in proteins.


 Please wait while we load your content...
Please wait while we load your content...