Stereospecific nucleophilic substitution at tertiary and quaternary stereocentres
Abstract
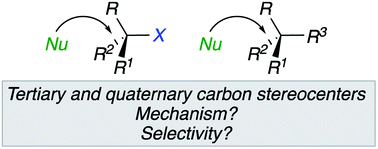
Nucleophilic substitution reactions have always been considered as one of the most powerful reactions for the creation of carbon–carbon or carbon–heteroatom bonds in organic synthesis. In contrast to secondary carbons, the steric shielding of tertiary carbons retards a concerted, stereospecific nucleophilic substitution, and ionizing pathways often lead to nonselective substitution due to ion pair dissociation. In this minireview, we will detail pioneering contributions and more recent achievements emphasizing the feasibility of nucleophilic substitution on tertiary stereocentres under certain conditions, with inversion of configuration. The development of these transformations at tertiary centres are of remarkable added value to practitioners in the field of complex molecule synthesis. A stereoselective substitution at a quaternary carbon stereocentre with inversion of configuration is also discussed in the case of a three-membered ring.


 Please wait while we load your content...
Please wait while we load your content...