Benzannulation of isobenzopyryliums with electron-rich alkynes: a modular access to β-functionalized naphthalenes†
Abstract
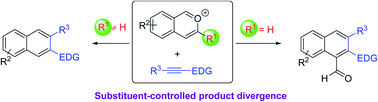
Described here is a modular strategy for the rapid synthesis of β-functionalized electron-rich naphthalenes, a family of valuable molecules lacking general access previously. Our approach employs an intermolecular benzannulation of in situ generated isobenzopyrylium ions with various electron-rich alkynes, which were not well utilized for this type of reaction before. These reactions not only feature a broad scope, complete regioselectivity, and mild conditions, but also exhibit unusual product divergence depending on the substrate substitution pattern. This divergence allows further expansion of the product diversity. Control experiments provided preliminary insights into the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...