Synthesis of cycloiptycenes from carbon nanobelts†
Abstract
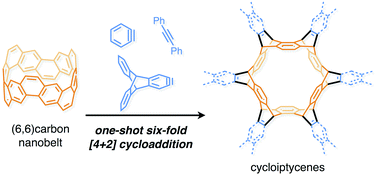
The synthesis of each of the cycloiptycene derivatives was achieved in one step from the (6,6)carbon nanobelt. It was revealed that the carbon nanobelt reacted as a diene in the Diels–Alder reaction with arynes and alkynes. The structures of all products were identified by X-ray crystallography to confirm that the Diels–Alder reactions took place at the six central benzene rings of the carbon nanobelt. DFT calculations indicated that the release of strain energy is the driving force to promote the Diels–Alder reaction. By using this method, we have successfully synthesized cyclotetracosiptycene, the largest iptycene ever synthesized.


 Please wait while we load your content...
Please wait while we load your content...