Self-adjusting binding pockets enhance H2 and CH4 adsorption in a uranium-based metal–organic framework†
Abstract
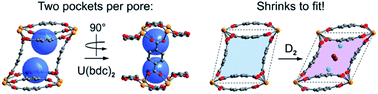
A new, air-stable, permanently porous uranium(IV) metal–organic framework U(bdc)2 (1, bdc2− = 1,4-benzenedicarboxylate) was synthesized and its H2 and CH4 adsorption properties were investigated. Low temperature adsorption isotherms confirm strong adsorption of both gases in the framework at low pressures. In situ gas-dosed neutron diffraction experiments with different D2 loadings revealed a rare example of cooperative framework contraction (ΔV = −7.8%), triggered by D2 adsorption at low pressures. This deformation creates two optimized binding pockets for hydrogen (Qst = −8.6 kJ mol−1) per pore, in agreement with H2 adsorption data. Analogous experiments with CD4 (Qst = −24.8 kJ mol−1) and N,N-dimethylformamide as guests revealed that the binding pockets in 1 adjust by selective framework contractions that are unique for each adsorbent, augmenting individual host–guest interactions. Our results suggest that the strategic combination of binding pockets and structural flexibility in metal–organic frameworks holds great potential for the development of new adsorbents with an enhanced substrate affinity.
- This article is part of the themed collections: 2020 Chemical Science HOT Article Collection and Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...