Single-atom replacement as a general approach towards visible-light/near-infrared heavy-atom-free photosensitizers for photodynamic therapy†
Abstract
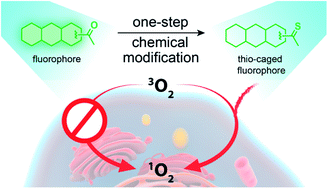
Photodynamic therapy has become an emerging strategy for the treatment of cancer. This technology relies on the development of photosensitizers (PSs) that convert molecular oxygen to cytotoxic reactive oxygen species upon exposure to light. In this study, we have developed a facile and general strategy for obtaining visible light/near-infrared-absorbing PSs by performing a simple sulfur-for-oxygen replacement within existing fluorophores. Thionation of carbonyl groups within existing fluorophore cores leads to an improvement of the singlet oxygen quantum yield and molar absorption coefficient at longer wavelengths (deep to 600–800 nm). Additionally, these thio-based PSs lack dark cytotoxicity but exhibit significant phototoxicity against monolayer cancer cells and 3D multicellular tumor spheroids with IC50 in the micromolar range. To achieve tumor-specific delivery, we have conjugated these thio-based PSs to an antibody and demonstrated their tumor-specific therapeutic activity.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...