Facile benzene reduction promoted by a synergistically coupled Cu–Co–Ce ternary mixed oxide†
Abstract
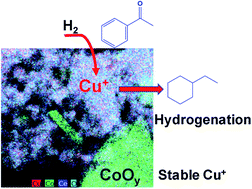
Hydrogenation of aromatic rings promoted by earth-abundant metal composites under mild conditions is an attractive and challenging subject in the long term. In this work, a simple active site creation and stabilization strategy was employed to obtain a Cu+-containing ternary mixed oxide catalyst. Simply by pre-treatment of the ternary metal oxide precursor under a H2 atmosphere, a Cu+-derived heterogeneous catalyst was obtained and denoted as Cu1Co5Ce5Ox. The catalyst showed (1) high Cu+ species content, (2) a uniform distribution of Cu+ doped into the lattices of CoOx and CeO2, (3) formation of CoOx/CuOx and CeO2/CuOx interfaces, and (4) a mesoporous structure. These unique properties of Cu1Co5Ce5Ox endow it with pretty high hydrogenation activity for aromatic rings under mild conditions (100 °C with 5 bar H2), which is much higher than that of the corresponding binary counterparts and even exceeds the performance of commercial noble metal catalysts (e.g. Pd/C). The synergetic effect plays a crucial role in the catalytic procedure with CeO2 functioning as a hydrogen dissociation and transfer medium, Cu+ hydrogenating the benzene ring and CoOx stabilizing the unstable Cu+ species. This will unlock a new opportunity to design highly efficient earth-abundant metal-derived heterogeneous catalysts via interface interactions.


 Please wait while we load your content...
Please wait while we load your content...