Intermolecular oxyarylation of olefins with aryl halides and TEMPOH catalyzed by the phenolate anion under visible light†
Abstract
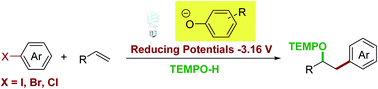
The phenolate anion was discovered as a new photocatalyst with strong reduction potentials. Under visible light irradiation, the phenolate anion enabled the reduction of (hetero)aryl halides (including electron-rich aryl chlorides) to (hetero)aryl radicals through single electron transfer. Based on this new photocatalyst, a novel and efficient photocatalytic protocol for the intermolecular oxyarylation of olefins with aryl halides and TEMPOH was developed. The developed three-component coupling reaction proceeded under redox-neutral reaction conditions with stable and readily available synthons and exhibited broad substrate scope. The utility of this process was further highlighted by the diversified chemical manipulation of the resulting oxyarylation products and the late-stage modification of active pharmaceutical ingredients.


 Please wait while we load your content...
Please wait while we load your content...