Discovery of a synthesis method for a difluoroglycine derivative based on a path generated by quantum chemical calculations†
Abstract
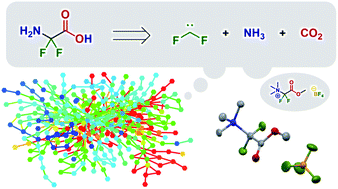
The systematic exploration of synthetic pathways to afford a desired product through quantum chemical calculations remains a considerable challenge. In 2013, Maeda et al. introduced ‘quantum chemistry aided retrosynthetic analysis’ (QCaRA), which uses quantum chemical calculations to search systematically for the decomposition paths of a target product and proposes a synthesis method. However, until now, no new reactions suggested by QCaRA have been reported to lead to experimental discoveries. Using a difluoroglycine derivative as a target, this study investigated the ability of QCaRA to suggest various synthetic paths to the target without relying on previous data or the knowledge and experience of chemists. Furthermore, experimental verification of the most promising path chosen by an organic chemist among the predicted paths led to the discovery of a synthesis method for a difluoroglycine derivative. We emphasize that the purpose of this study is not to propose a fully automated workflow. Therefore, the extent of the hands-on expertise of chemists required during the verification process was also evaluated. These insights are expected to advance the applicability of QCaRA to the discovery of viable experimental synthetic routes.
- This article is part of the themed collections: Celebrating 10 years of Chemical Science and Accelerating Chemistry Symposium Collection


 Please wait while we load your content...
Please wait while we load your content...