Ring expansion of alumoles with organic azides: selective formation of six-membered aluminum–nitrogen heterocycles†
Abstract
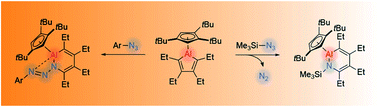
Aside from simple Lewis acid–base chemistry, the reaction chemistry of aluminacyclopentadienes, which are commonly referred to as aluminoles or simply alumoles, remains relatively underdeveloped. To date, few attempts to extend their inherent insertion and cycloaddition reactivity to the construction of stable aluminum-containing heterocycles have been reported. Herein, we demonstrate the selective ring expansion of a cyclopentadienyl-substituted alumole with a series of organic azides to form unsaturated six-membered AlN heterocycles. Depending on the substituent on the azide, the reaction proceeds either with or without loss of dinitrogen, leading to incorporation of only the “NR” unit of the azide or the entire azo substituent into the periphery of the heterocycle. A deeper understanding of these ring expansion reactions is reached through computational studies, illustrating deviations in the mechanism as a function of the organic azide employed.


 Please wait while we load your content...
Please wait while we load your content...