Quinone methide dimers lacking labile hydrogen atoms are surprisingly excellent radical-trapping antioxidants†
Abstract
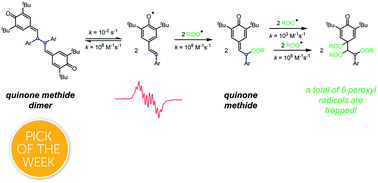
Hydrogen atom transfer (HAT) is the mechanism by which the vast majority of radical-trapping antioxidants (RTAs), such as hindered phenols, inhibit autoxidation. As such, at least one weak O–H bond is the key structural feature which underlies the reactivity of phenolic RTAs. We recently observed that quinone methide dimers (QMDs) synthesized from hindered phenols are significantly more reactive RTAs than the phenols themselves despite lacking O–H bonds. Herein we describe our efforts to elucidate the mechanism by which they inhibit autoxidation. Four possible reaction paths were considered: (1) HAT from the C–H bonds on the carbon atoms which link the quinone methide moieties; (2) tautomerization or hydration of the quinone methide(s) in situ followed by HAT from the resultant phenolic O–H; (3) direct addition of peroxyl radicals to the quinone methide(s), and (4) homolysis of the weak central C–C bond in the QMD followed by combination of the resultant persistent phenoxyl radicals with peroxyl radicals. The insensitivity of the reactivity of the QMDs to substituent effects, solvent effects and a lack of kinetic isotope effects rule out the HAT reactions (mechanisms 1 and 2). Simple (monomeric) quinone methides, to which peroxyl radicals add, were found to be ca. 100-fold less reactive than the QMDs, ruling out mechanism 3. These facts, combined with the poor RTA activity we observe for a QMD with a stronger central C–C bond, support mechanism 4. The lack of solvent effects on the RTA activity of QMDs suggests that they may find application as additives to materials which contain H-bonding accepting moieties that can dramatically suppress the reactivity of conventional RTAs, such as phenols. This reactivity does not extend to biological membranes owing to the increased microviscosity of the phospholipid bilayer, which suppresses QMD dissociation in favour of recombination. Interestingly, the simple QMs were found to be very good RTAs in phospholipid bilayers – besting even the most potent form of vitamin E.
- This article is part of the themed collections: 2020 ChemSci Pick of the Week Collection and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...