Macrocycle synthesis strategy based on step-wise “adding and reacting” three components enables screening of large combinatorial libraries†
Abstract
Macrocycles provide an attractive modality for drug development, but generating ligands for new targets is hampered by the limited availability of large macrocycle libraries. We have established a solution-phase macrocycle synthesis strategy in which three building blocks are coupled sequentially in efficient alkylation reactions that eliminate the need for product purification. We demonstrate the power of the approach by combinatorially reacting 15 bromoacetamide-activated tripeptides, 42 amines, and 6 bis-electrophile cyclization linkers to generate a 3780-compound library with minimal effort. Screening against thrombin yielded a potent and selective inhibitor (Ki = 4.2 ± 0.8 nM) that efficiently blocked blood coagulation in human plasma. Structure–activity relationship and X-ray crystallography analysis revealed that two of the three building blocks acted synergistically and underscored the importance of combinatorial screening in macrocycle development. The three-component library synthesis approach is general and offers a promising avenue to generate macrocycle ligands to other targets.
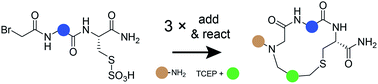


 Please wait while we load your content...
Please wait while we load your content...