Transformation from helical to layered supramolecular organization of asymmetric perylene diimides via multiple intermolecular hydrogen bonding†
Abstract
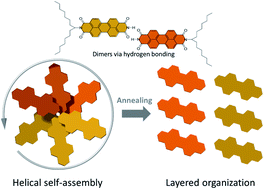
The self-assembly of an asymmetric perylene diimide (PDI) with a dove-tailed side chain is investigated after thermal annealing at various temperatures in solid-state. After annealing at low temperatures PDI dimers are formed through hydrogen bonding between the imide and carbonyl groups, together with π–stacking interactions leading to a helical packing of the molecules in supramolecular columnar structures. After annealing at higher temperatures a transformation into a layered organization with improved molecular order is observed. The driving force for this thermodynamically favorable reorganization and planarization of the PDI dimer is additional intermolecular hydrogen bonding between the carbonyl and aromatic H(Ar) that is also present in monolayers that are visualized by scanning tunneling microscopy. This study demonstrates the importance of hydrogen bonding on tuning the supramolecular organization of asymmetric PDI. Depending on the dominating interactions, a wide variety of complex supramolecular structures with unique properties can be initiated.


 Please wait while we load your content...
Please wait while we load your content...