Carboxylate breaks the arene C–H bond via a hydrogen-atom-transfer mechanism in electrochemical cobalt catalysis†
Abstract
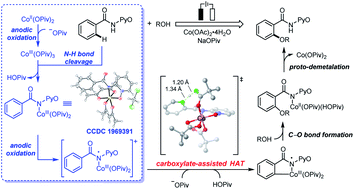
Combined computational and experimental studies elucidated the distinctive mechanistic features of electrochemical cobalt-catalyzed C–H oxygenation. A sequential electrochemical–chemical (EC) process was identified for the formation of an amidylcobalt(III) intermediate. The synthesis, characterization, cyclic voltammetry studies, and stoichiometric reactions of the related amidylcobalt(III) intermediate suggested that a second on-cycle electro-oxidation occurs on the amidylcobalt(III) species, which leads to a formal Co(IV) intermediate. This amidylcobalt(IV) intermediate is essentially a cobalt(III) complex with one additional single electron distributed on the coordinating heteroatoms. The radical nature of the coordinating pivalate allows the formal Co(IV) intermediate to undergo a novel carboxylate-assisted HAT mechanism to cleave the arene C–H bond, and a CMD mechanism could be excluded for a Co(III/I) catalytic scenario. The mechanistic understanding of electrochemical cobalt-catalyzed C–H bond activation highlights the multi-tasking electro-oxidation and the underexplored reaction channels in electrochemical transition metal catalysis.


 Please wait while we load your content...
Please wait while we load your content...