Introduction of a 7-aza-6-MeO-indoline auxiliary in Lewis-acid/photoredox cooperative catalysis: highly enantioselective aminomethylation of α,β-unsaturated amides†
Abstract
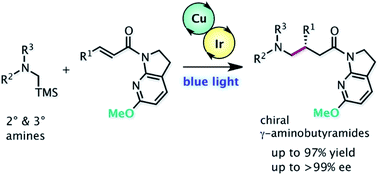
An efficient cooperative chiral Lewis acid/photoredox catalytic system for engaging highly reactive radicals in highly enantioselective conjugate addition to α,β-unsaturated carbonyls is highly desirable. Direct photoexcitation of unbound substrates typically induces undesired background pathways for racemic products and remains a formidable challenge to be addressed in the area of enantioselective photocatalysis. Herein, we report a cooperative catalytic system comprising a chiral Cu(I) complex and an Ir(III) photocatalyst fueled by visible-light irradiation that allows for seamless integration of the catalytic formation of α-amino alkyl radicals and subsequent enantioselective addition to α,β-unsaturated amides. A 7-aza-6-MeO-indoline attachment on the amide substrates plays a pivotal role in suppressing the undesired pathways, resulting in excellent enantioselectivity and enabling expedited access to valuable γ-aminobutyramides. The indoline amide was readily diversified with full recovery of the azaindoline attachment, highlighting the synthetic utility of this cooperative catalytic system.


 Please wait while we load your content...
Please wait while we load your content...