Direct regioisomer analysis of crude reaction mixtures via molecular rotational resonance (MRR) spectroscopy†
Abstract
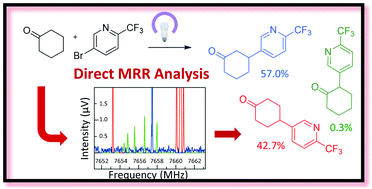
Direct analyses of crude reaction mixtures have been carried out using molecular rotational resonance (MRR) spectroscopy. Two examples are presented, a demonstration application in photocatalytic CH-arylation as well as generation of an intermediate in a natural product synthesis. In both cases, the reaction can proceed at more than one site, leading to a mixture of regioisomers that can be challenging to distinguish. MRR structural parameters were calculated for the low lying conformers for the desired compounds, and then compared to the experimental spectra of the crude mixtures to confirm the presence of these species. Next, quantitation was performed by comparing experimentally measured line intensities with simulations based on computed values for the magnitude and direction of the molecular dipole moment of each species. This identification and quantification was performed without sample purification and without isolated standards of the compounds of interest. The values obtained for MRR quantitation were in good agreement with the chromatographic values. Finally, previously unknown impurities were discovered within the photocatalytic CH-arylation work. This paper demonstrates the utility of MRR as a reaction characterization tool to simplify analytical workflows.
- This article is part of the themed collection: Sustainable synthesis and catalysis – Chemical Science symposium collection


 Please wait while we load your content...
Please wait while we load your content...