Structure and redox tuning of gas adsorption properties in calixarene-supported Fe(ii)-based porous cages†
Abstract
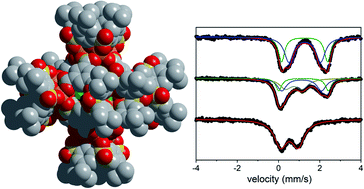
We describe the synthesis of Fe(II)-based octahedral coordination cages supported by calixarene capping ligands. The most porous of these molecular cages has an argon accessible BET surface area of 898 m2 g−1 (1497 m2 g−1 Langmuir). The modular synthesis of molecular cages allows for straightforward substitution of both the bridging carboxylic acid ligands and the calixarene caps to tune material properties. In this context, the adsorption enthalpies of C2/C3 hydrocarbons ranged from −24 to −46 kJ mol−1 at low coverage, where facile structural modifications substantially influence hydrocarbon uptakes. These materials exhibit remarkable stability toward oxidation or decomposition in the presence of air and moisture, but application of a suitable chemical oxidant generates oxidized cages over a controlled range of redox states. This provides an additional handle for tuning the porosity and stability of the Fe cages.


 Please wait while we load your content...
Please wait while we load your content...