Coordination polymer glass from a protic ionic liquid: proton conductivity and mechanical properties as an electrolyte†
Abstract
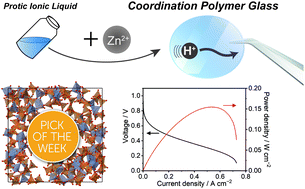
High proton conducting electrolytes with mechanical moldability are a key material for energy devices. We propose an approach for creating a coordination polymer (CP) glass from a protic ionic liquid for a solid-state anhydrous proton conductor. A protic ionic liquid (dema)(H2PO4), with components which also act as bridging ligands, was applied to construct a CP glass (dema)0.35[Zn(H2PO4)2.35(H3PO4)0.65]. The structural analysis revealed that large Zn–H2PO4−/H3PO4 coordination networks formed in the CP glass. The network formation results in enhancement of the properties of proton conductivity and viscoelasticity. High anhydrous proton conductivity (σ = 13.3 mS cm−1 at 120 °C) and a high transport number of the proton (0.94) were achieved by the coordination networks. A fuel cell with this CP glass membrane exhibits a high open-circuit voltage and power density (0.15 W cm−2) under dry conditions at 120 °C due to the conducting properties and mechanical properties of the CP glass.
- This article is part of the themed collection: 2020 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...