A solvent-dependent chirality-switchable thia-Michael addition to α,β-unsaturated carboxylic acids using a chiral multifunctional thiourea catalyst†
Abstract
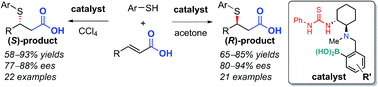
An asymmetric thia-Michael addition of arylthiols to α,β-unsaturated carboxylic acids using a thiourea catalyst that bears arylboronic acid and tertiary amine moieties is reported. Both enantiomers of the Michael adducts can be obtained in high enantioselectivity and good yield merely by changing the solvent. The origin of the chirality switch in the products was examined in each solvent via spectroscopic analyses.


 Please wait while we load your content...
Please wait while we load your content...