The role of porphyrin peripheral substituents in determining the reactivities of ferrous nitrosyl species†
Abstract
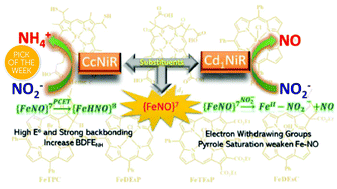
Ferrous nitrosyl {FeNO}7 species is an intermediate common to the catalytic cycles of Cd1NiR and CcNiR, two heme-based nitrite reductases (NiR), and its reactivity varies dramatically in these enzymes. The former reduces NO2− to NO in the denitrification pathway while the latter reduces NO2− to NH4+ in a dissimilatory nitrite reduction. With very similar electron transfer partners and heme based active sites, the origin of this difference in reactivity has remained unexplained. Differences in the structure of the heme d1 (Cd1NiR), which bears electron-withdrawing groups and has saturated pyrroles, relative to heme c (CcNiR) are often invoked to explain these reactivities. A series of iron porphyrinoids, designed to model the electron-withdrawing peripheral substitution as well as the saturation present in heme d1 in Cd1NiR, and their NO adducts were synthesized and their properties were investigated. The data clearly show that the presence of electron-withdrawing groups (EWGs) and saturated pyrroles together in a synthetic porphyrinoid (FeDEsC) weakens the Fe–NO bond in {FeNO}7 adducts along with decreasing the bond dissociation free energies (BDFENH) of the {FeHNO}8 species. The EWG raises the E° of the {FeNO}7/8 process, making the electron transfer (ET) facile, but decreases the pKa of {FeNO}8 species, making protonation (PT) difficult, while saturation has the opposite effect. The weakening of the Fe–NO bonding biases the {FeNO}7 species of FeDEsC for NO dissociation, as in Cd1NiR, which is otherwise set-up for a proton-coupled electron transfer (PCET) to form an {FeHNO}8 species eventually leading to its further reduction to NH4+.
- This article is part of the themed collections: 2020 ChemSci Pick of the Week Collection and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...