CuA-based chimeric T1 copper sites allow for independent modulation of reorganization energy and reduction potential†
Abstract
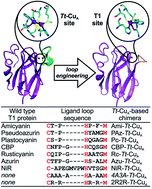
Attaining rational modulation of thermodynamic and kinetic redox parameters of metalloproteins is a key milestone towards the (re)design of proteins with new or improved redox functions. Here we report that implantation of ligand loops from natural T1 proteins into the scaffold of a CuA protein leads to a series of distorted T1-like sites that allow for independent modulation of reduction potentials (E°′) and electron transfer reorganization energies (λ). On the one hand E°′ values could be fine-tuned over 120 mV without affecting λ. On the other, λ values could be modulated by more than a factor of two while affecting E°′ only by a few millivolts. These results are in sharp contrast to previous studies that used T1 cupredoxin folds, thus highlighting the importance of the protein scaffold in determining such parameters.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...