Nickel catalysis enables convergent paired electrolysis for direct arylation of benzylic C–H bonds†
Abstract
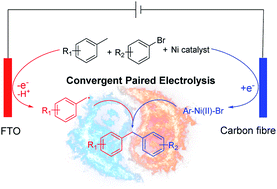
Convergent paired electrosynthesis is an energy-efficient approach in organic synthesis; however, it is limited by the difficulty to match the innate redox properties of reaction partners. Here we use nickel catalysis to cross-couple the two intermediates generated at the two opposite electrodes of an electrochemical cell, achieving direct arylation of benzylic C–H bonds. This method yields a diverse set of diarylmethanes, which are important structural motifs in medicinal and materials chemistry. Preliminary mechanistic study suggests oxidation of a benzylic C–H bond, Ni-catalyzed C–C coupling, and reduction of a Ni intermediate as key elements of the catalytic cycle.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...