Bimetallic metal–organic frameworks and their derivatives
Abstract
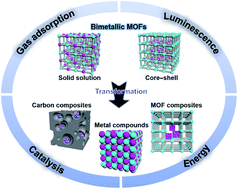
Bimetallic metal–organic frameworks (MOFs) have two different metal ions in the inorganic nodes. According to the metal distribution, the architecture of bimetallic MOFs can be classified into two main categories namely solid solution and core–shell structures. Various strategies have been developed to prepare bimetallic MOFs with controlled compositions and structures. Bimetallic MOFs show a synergistic effect and enhanced properties compared to their monometallic counterparts and have found many applications in the fields of gas adsorption, catalysis, energy storage and conversion, and luminescence sensing. Moreover, bimetallic MOFs can serve as excellent precursors/templates for the synthesis of functional nanomaterials with controlled sizes, compositions, and structures. Bimetallic MOF derivatives show exposed active sites, good stability and conductivity, enabling them to extend their applications to the catalysis of more challenging reactions and electrochemical energy storage and conversion. This review provides an overview of the significant advances in the development of bimetallic MOFs and their derivatives with special emphases on their preparation and applications.
- This article is part of the themed collections: Celebrating 15 years of exceptional research in Chemical Science and Most popular 2019-2020 review articles


 Please wait while we load your content...
Please wait while we load your content...