Redox-inactive ions control the redox-activity of molecular vanadium oxides†
Abstract
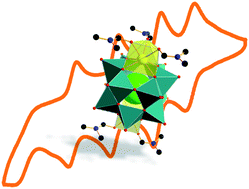
Polyoxometalates are key materials for energy conversion and storage due to their unique chemical tunability and electrochemical reactivity. Herein, we report that functionalization of molecular vanadium oxides, polyoxovanadates, with redox-inert Ca2+ cations leads to a significant increase in their electron storage capabilities. The electrochemical performance of the Ca2+-functionalized dodecavanadate [Ca2V12O32Cl(DMF)3]2− (={Ca2V12}) was thus compared with that of the precursor compound (H2NMe2)2[V12O32Cl]3− (={V12}). {Ca2V12} can store up to five electrons per cluster, while {V12} only shows one reversible redox transition. In initial studies, we demonstrated that {Ca2V12} can be used as an active material in lithium-ion cathodes. Our results show how redox-inert cations can be used as structural and electrostatic stabilizers, leading to major changes in the redox-chemistry of polyoxovanadates.


 Please wait while we load your content...
Please wait while we load your content...